FDA cleared for ophthalmic use.
Eye clinics still use non-ophthalmic needles.
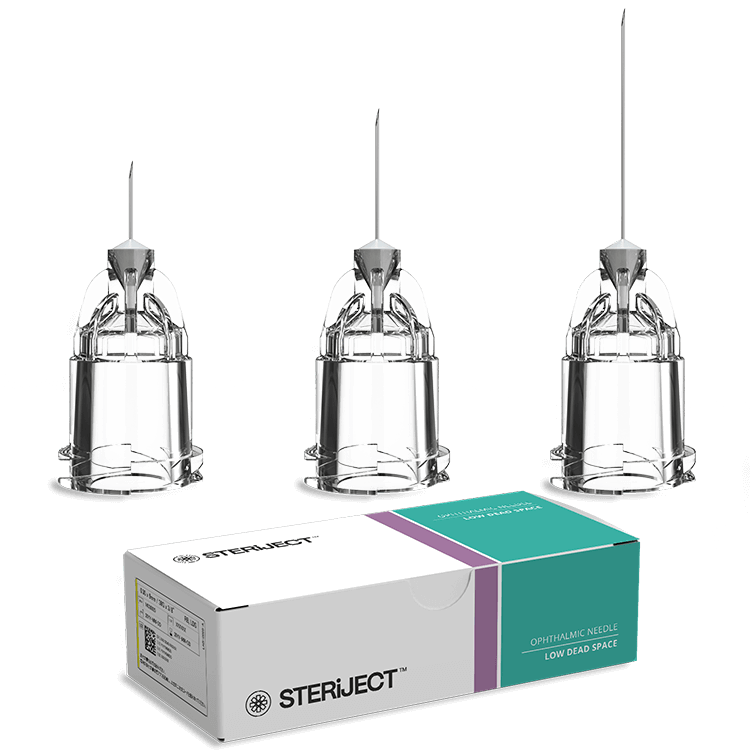
The sharp increase in retinal conditions such as neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), diabetic retinopathy, and retinal vein occlusion has led to a surge in intravitreal injection procedures. Despite this, many practices still rely on hypodermic needles not originally intended for use in the eye. STERiJECT™ was created to meet this unmet clinical need—offering a dedicated device for ophthalmologists and retina specialists that prioritizes dose control, patient comfort, and sterility.
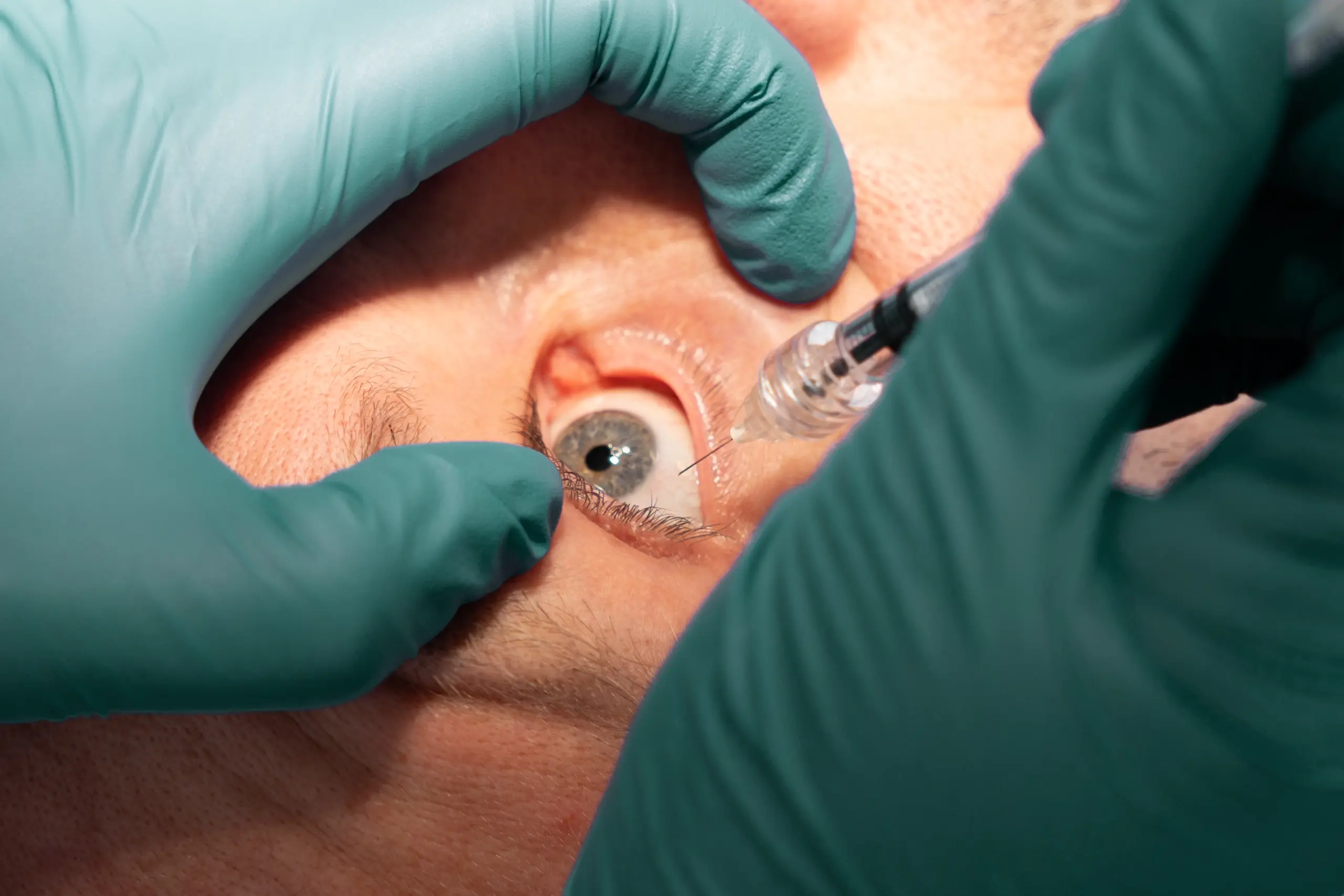
Regular Hypodermic Needle
Non compliant
Not tested
Endotoxin limit 20.0 EU/device
~ 0.04 ml

STERiJECT Ophthalmic Needle
FDA 510(k) issued
USP 789 compliant
Endotoxin limit 0.2 EU/device
~ 0.0 ml
Order STERiJECT Ophthalmic LDS Needles
Buy nowOptimal patient comfort.
STERiJECT™ ophthalmic needles are available in sizes ranging from 30G to 34G,
offering a balance between precision and patient comfort. These fine-gauge options
are designed specifically for intravitreal injections, where minimizing tissue
disruption is essential.
The needle design helps reduce the risk of vitreous reflux and subconjunctival hemorrhage,
while maintaining an optimal flow rate and injection force. This ensures consistent
drug delivery with minimal discomfort, even during high-volume clinical use.



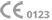 | TSK Laboratory International
©
| TSK Laboratory International
©

