The race is on around the globe to develop a vaccine for COVID-19 (SARS-CoV2), with multiple programmes, in multiple countries, actively developing and testing different solutions.
The unprecedented speed with which research and progress is being made in finding a safe, effective, and functioning vaccine means that hopefully it will not be too long before we will soon see one approved for use. This will have a major impact around the world and finally provide a resolution to a pandemic which continues to decimate populations across all continents.
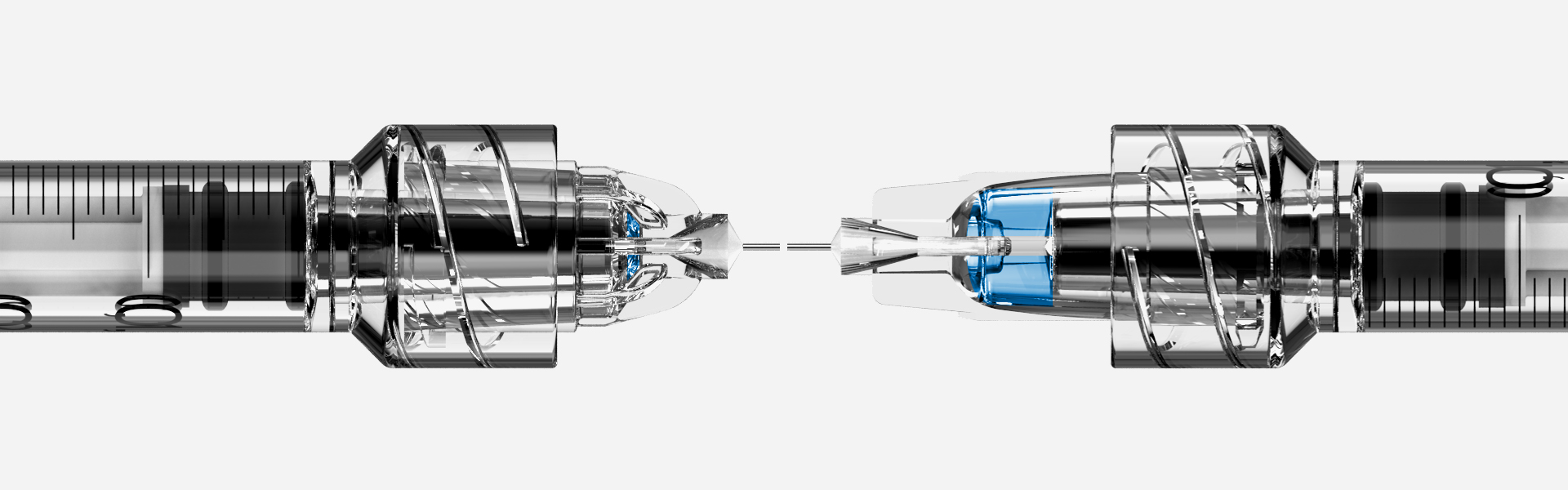
Governments and not-for-profit medical organisations are keen to invest, and invest heavily, to build up adequate supplies and orders from those pharmaceutical companies and partnerships which are beginning to show the most potential at making it to the finish line with a vaccine which can be delivered en masse, at national and global levels.[i] Of course, when the roll-out occurs, it will be unlike anything we have ever seen before with potentially billions of doses required to be administered, on an annual basis. Medical authorities, policy makers, and vaccine manufacturers need to think carefully about the ways in which mass vaccination of this kind can be delivered expediently, and cost-effectively, to the population and must consider the best tools for the job.

 | TSK Laboratory International
©
| TSK Laboratory International
©







